← contract risk management report Contract risk assessment checklist: 10 steps to follow report summary business professional Report business template format templates examples templatelab kb →
If you are searching about Risk Management Plan (RMP) | Pharmaceuticals and Medical Devices Agency you've visit to the right page. We have 35 Pics about Risk Management Plan (RMP) | Pharmaceuticals and Medical Devices Agency like Risk Management for Medical Devices: ISO 14971:2019 | Kvalito, Preparing a Medical Device Risk Management Review and Report and also Documenting Medical Device Risk Management through the Risk. Here you go:
Risk Management Plan (RMP) | Pharmaceuticals And Medical Devices Agency
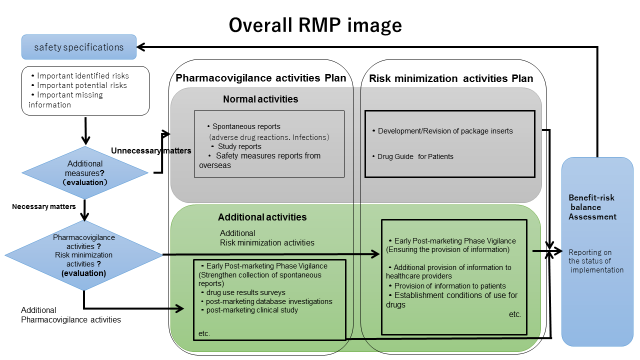 www.pmda.go.jp
www.pmda.go.jp
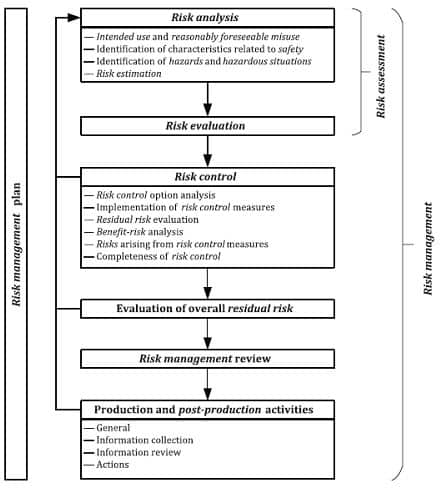
Case Study — Risk Management For Medical Devices (based On ISO 14971
 www.semanticscholar.org
www.semanticscholar.org
ISO 14971: Managing Risk In Medical Device Development
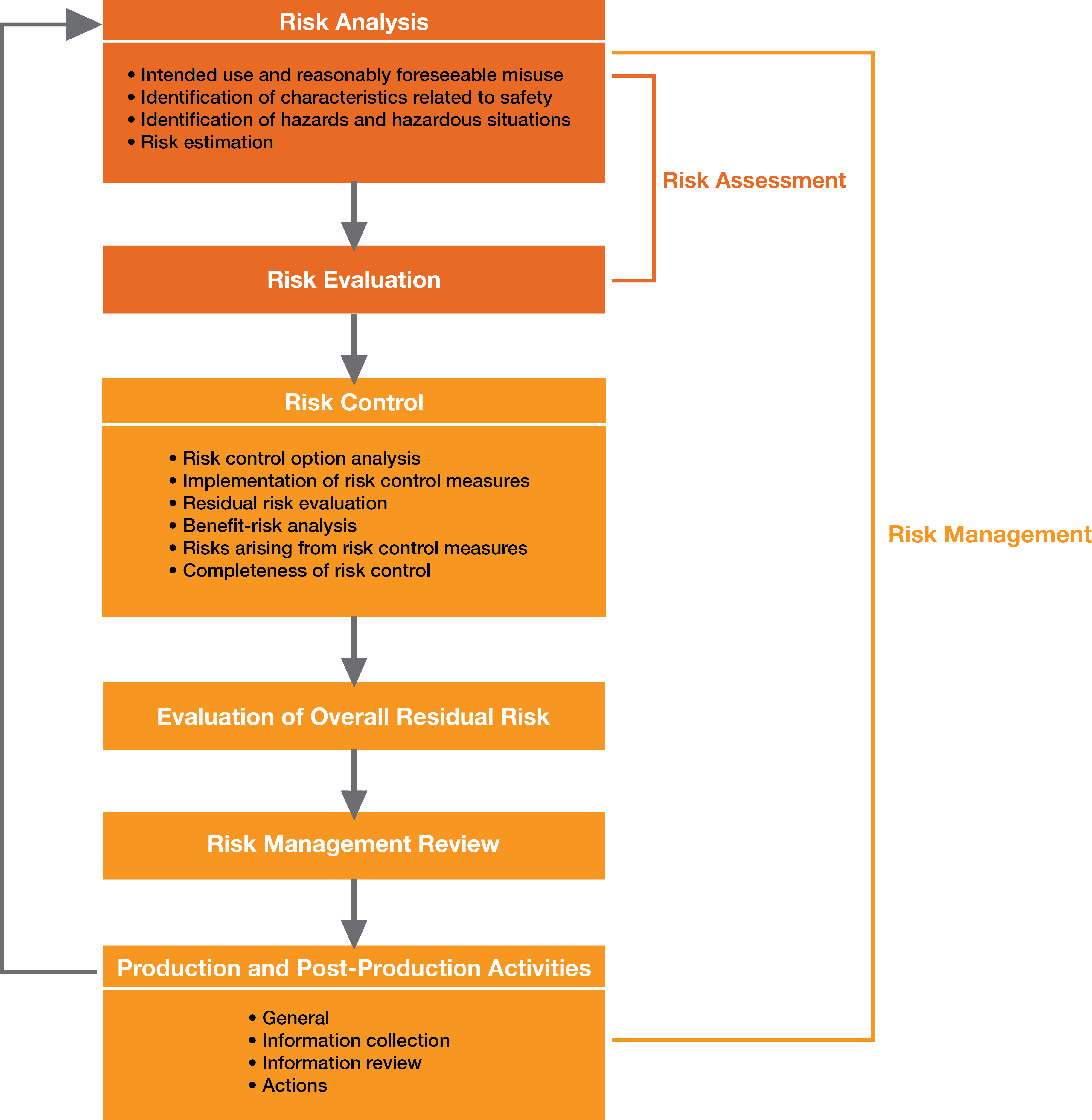 simbex.com
simbex.com
risk management process medical device iso flow chart 2021 representation schematic figure managing
Why Medical Device Risk Management Is As Complex As It Is Crucial
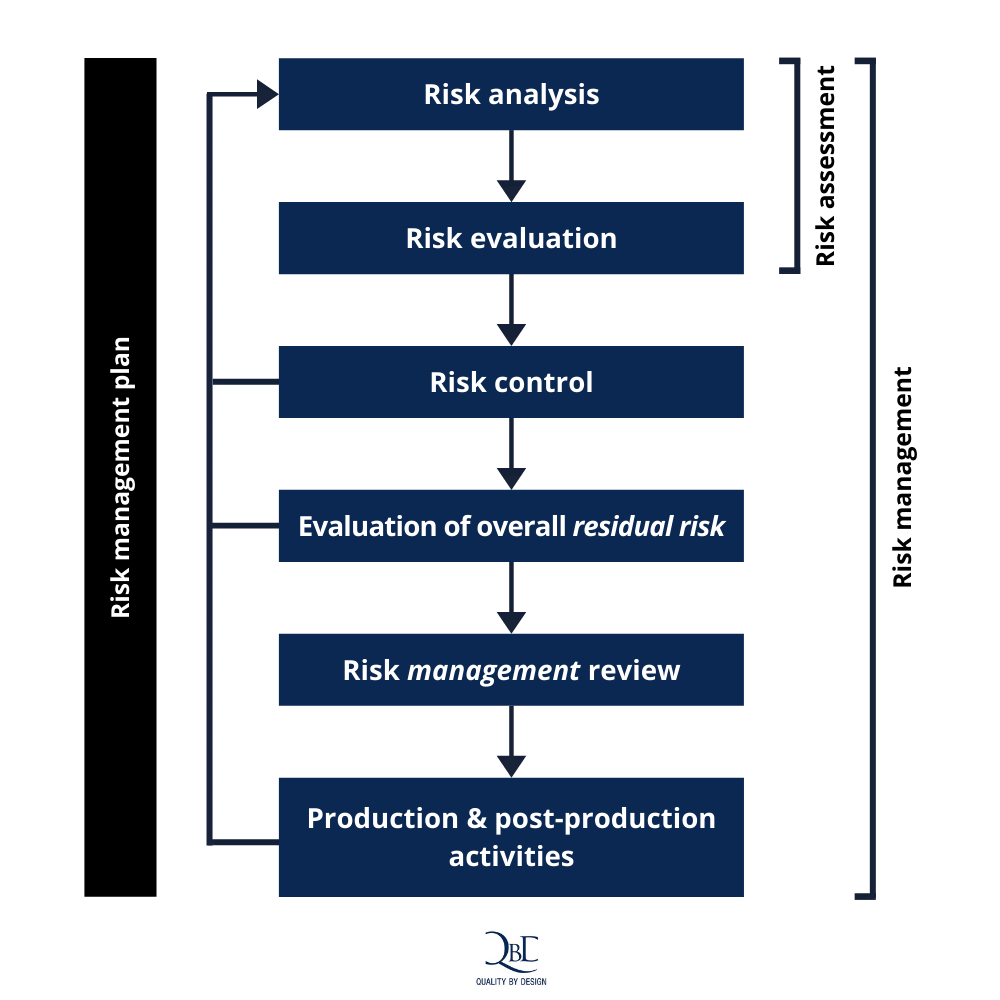 qbd.eu
qbd.eu
device crucial schematic representation
Risk Management In The Medical Device Industry In The EU - YouTube
 www.youtube.com
www.youtube.com
Navigating The Universe Of Risk In Medical Device Development
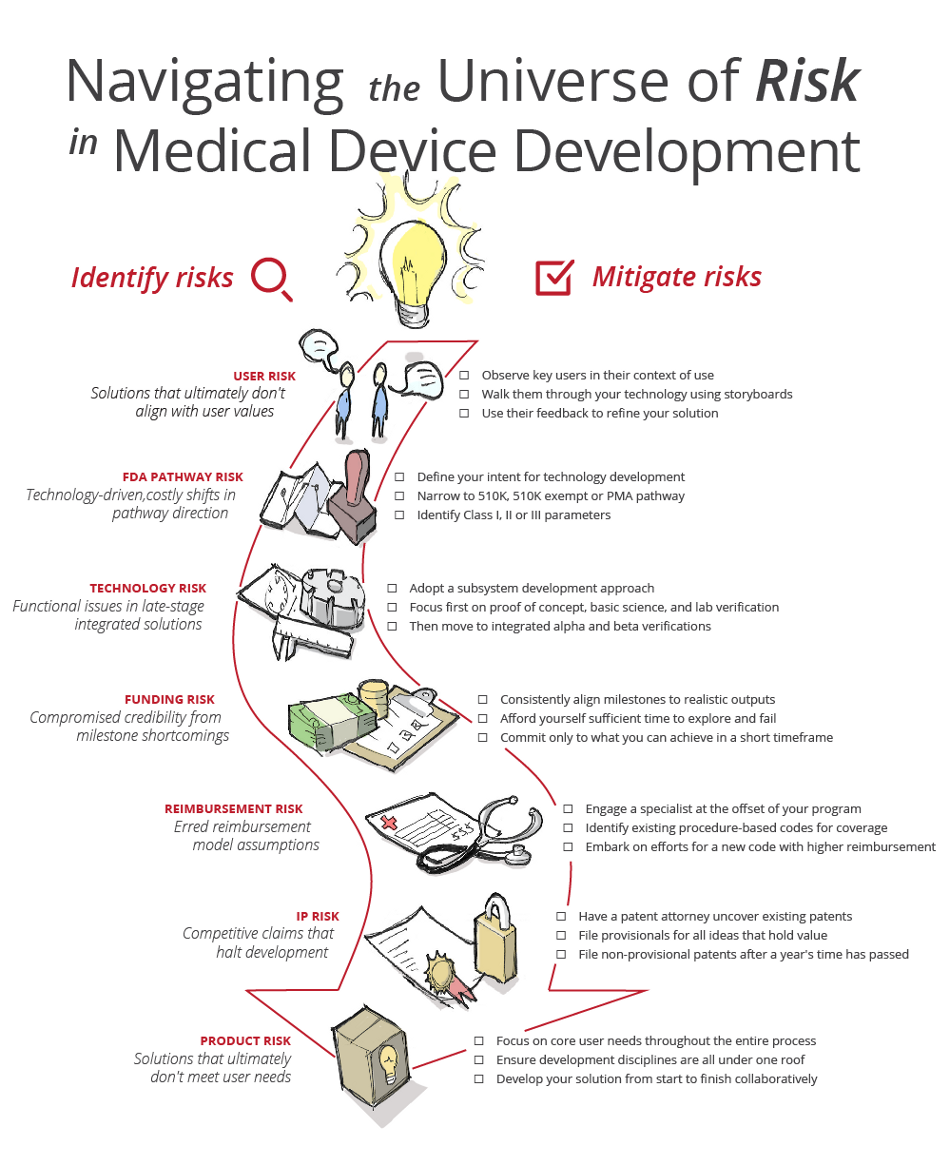 www.meddeviceonline.com
www.meddeviceonline.com
risk medical device development universe navigating infographic mitigation user
ISO 14971:2019 Medical Devices - Application Of Risk Management To
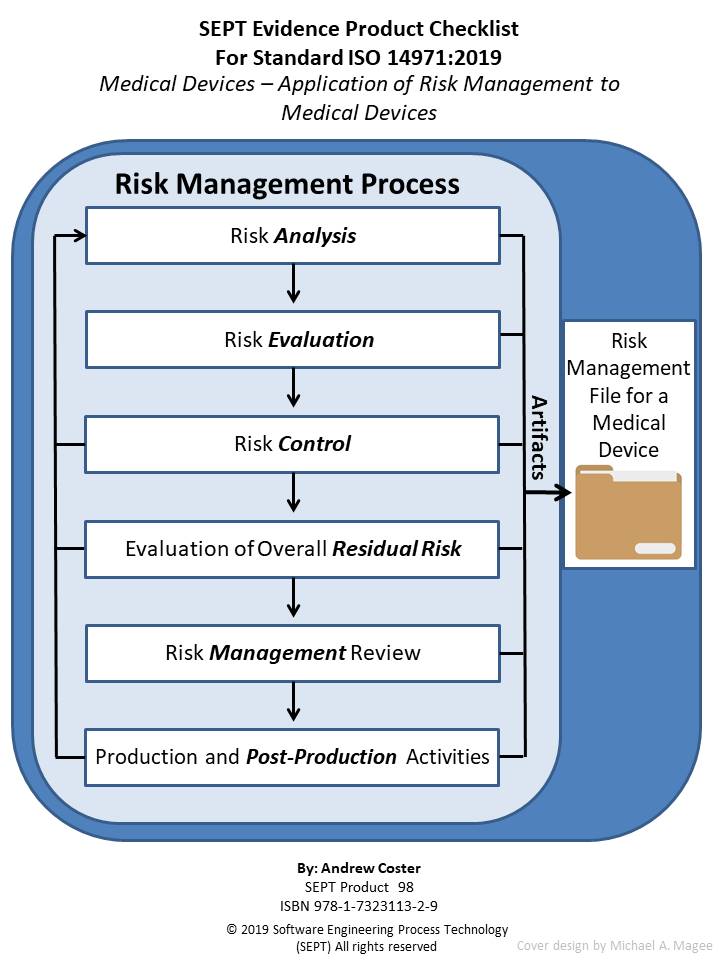 www.complianceonline.com
www.complianceonline.com
risk devices checklist optimize guidance detailed iec
Documenting Medical Device Risk Management Through The Risk
 array.aami.org
array.aami.org
Understanding ISO 14971 Medical Device Risk Management
 www.greenlight.guru
www.greenlight.guru
greenlight
Preparing A Medical Device Risk Management Review And Report
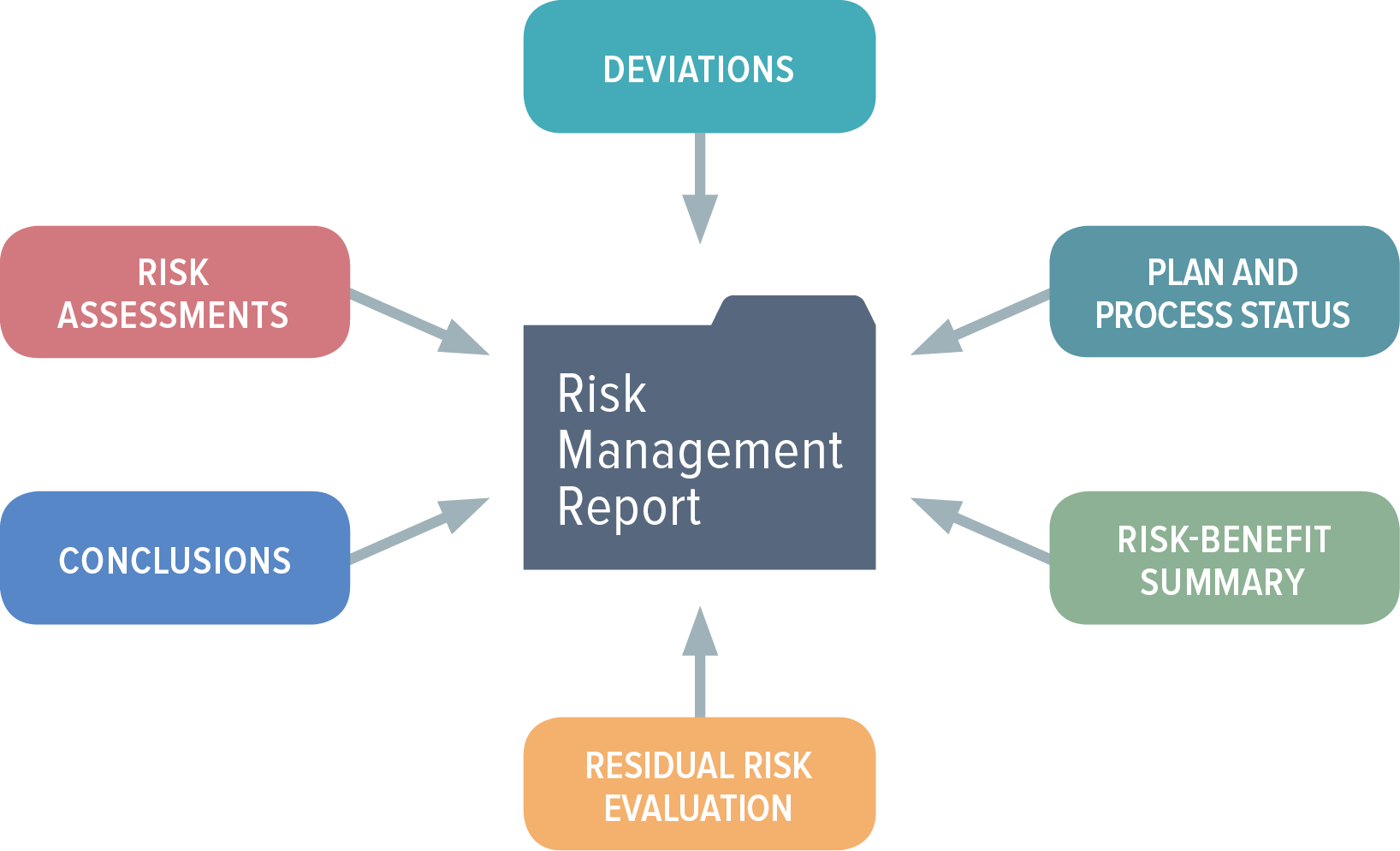 www.orielstat.com
www.orielstat.com
risk management report medical device review reporting iso part analysis planning tools control post
Risk Management In Medical Device | RS NESS
 rs-ness.com
rs-ness.com
01 Risk Management Report | PDF | Medical Device | Risk Management
 www.scribd.com
www.scribd.com
CAPA Procedure Template (ISO 13485, Medical Device) - Medical Device HQ
 medicaldevicehq.com
medicaldevicehq.com
Medical Device Risk Management Report Example - Template 2 : Resume
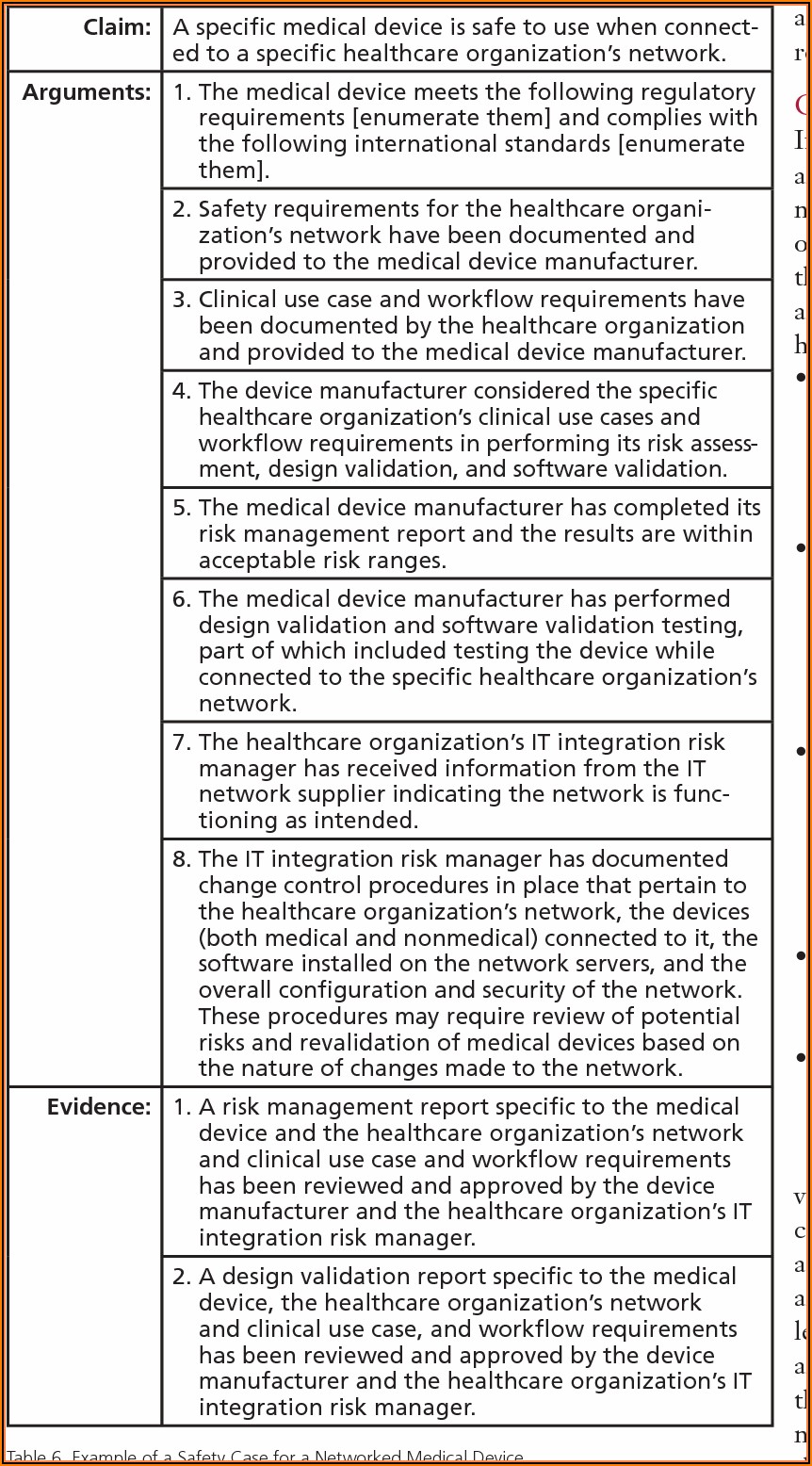 www.contrapositionmagazine.com
www.contrapositionmagazine.com
risk medical device management report example template google twitter share examples
The Big Picture For Medical Device Risk Management - Sunstone Pilot, Inc.
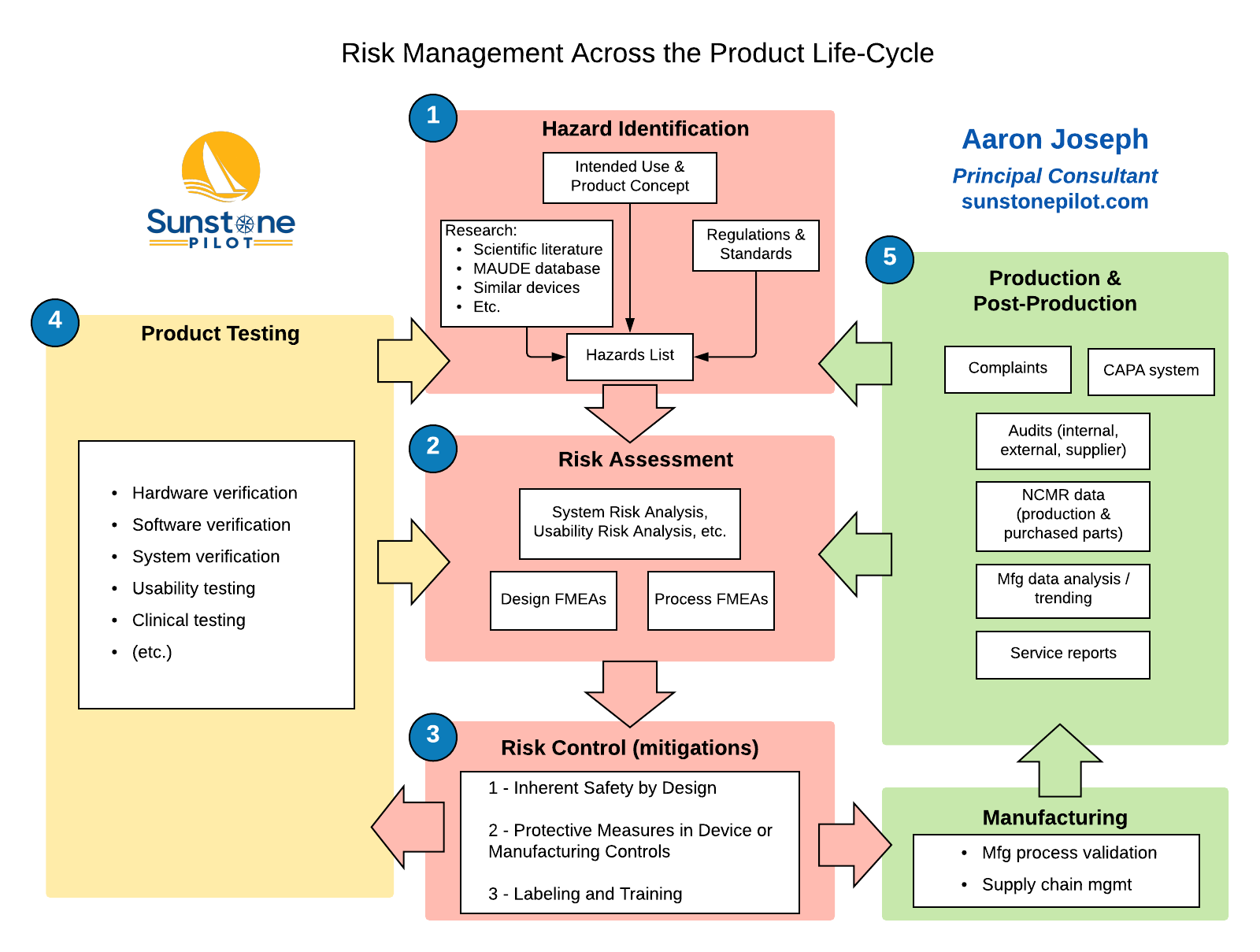 sunstonepilot.com
sunstonepilot.com
Understanding Medical Device Risk Management & ISO 14971
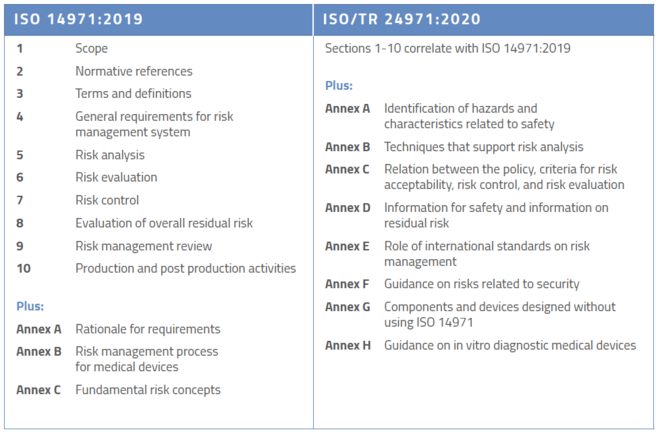 www.orielstat.com
www.orielstat.com
risk matrix procedure
RISK MANAGEMENT Report Example | PDF | Surgical Suture | Medical Device
 www.scribd.com
www.scribd.com
Medical Device Risk Management Report Template - Template 2 : Resume
 www.contrapositionmagazine.com
www.contrapositionmagazine.com
template risk report device medical management mitigation ecommerce plan business website example google twitter share professional
Medical Device Risk Management Report Consultants - I3CGlobal
 www.i3cglobal.com
www.i3cglobal.com
FMEA Vs ISO 14971 - Medical Device HQ 1
 medicaldevicehq.com
medicaldevicehq.com
ISO 14971 Medical Device Risk Management | Oriel STAT A MATRIX
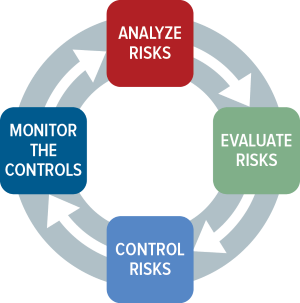 www.orielstat.com
www.orielstat.com
iso defined
Risk Management For Medical Device And ISO 14971:2019 Infographic
 medicaldevicehq.com
medicaldevicehq.com
Managing Risk For Medical Device Clinical Trials
 www.meddeviceonline.com
www.meddeviceonline.com
risk medical device management clinical trials iso process managing evaluation fig
Choosing The Right Medical Device Risk Management Tools
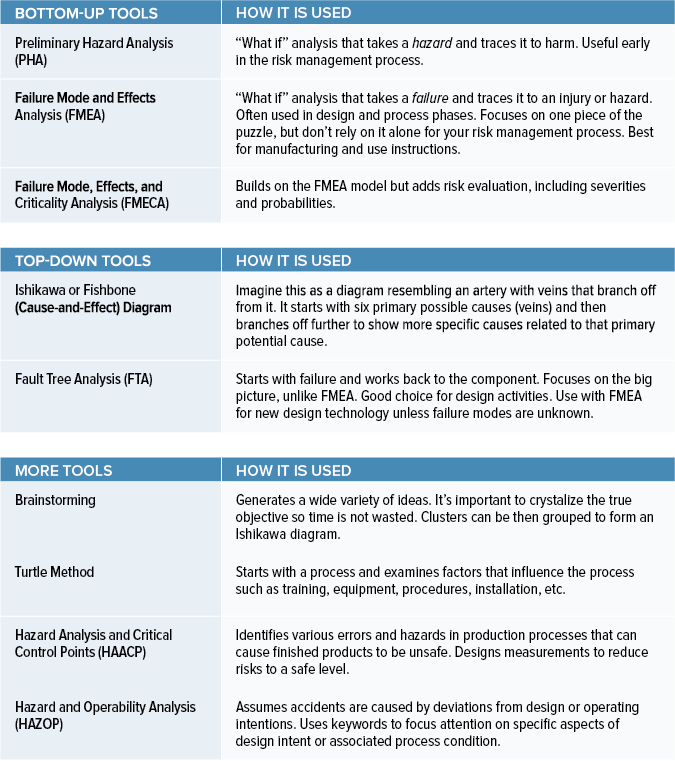 www.orielstat.com
www.orielstat.com
medical evaluation residual avoiding paralysis
Risk Management For Medical Devices As Defined By ISO 14971 | Risk
 www.pinterest.nz
www.pinterest.nz
iso resiko manajemen medis peralatan sesuai strategies
IMSXpress ISO 14971 Medical Device Risk Management And Hazard Analysis
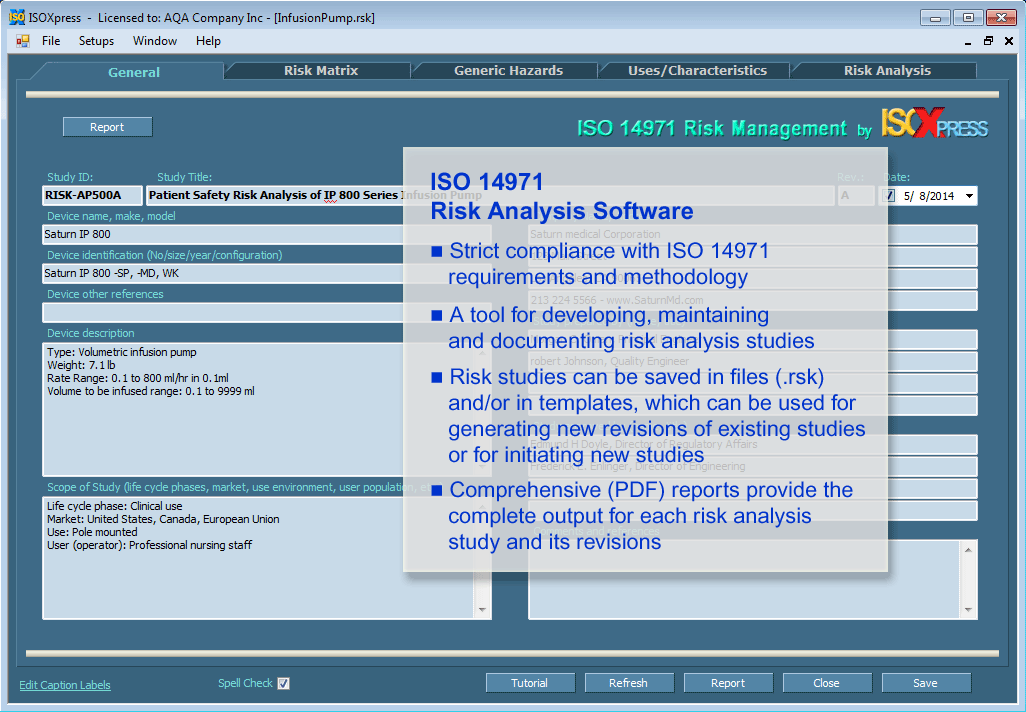 imsxp.com
imsxp.com
medical risk device management iso hazard analysis software modules
Risk Management Under EU Medical Device Regulation – CRITICAL CATALYST
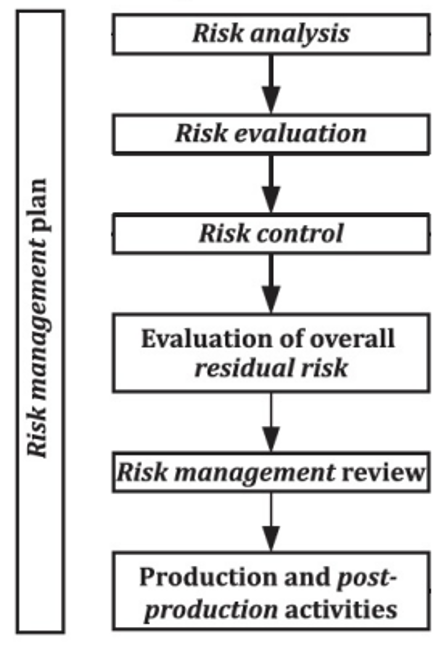 criticalcatalyst.com
criticalcatalyst.com
Risk Management For Medical Devices: ISO 14971:2019 | Kvalito
 kvalito.ch
kvalito.ch
iso include
ISO 14971 Medical Device Risk Management | Oriel STAT A MATRIX
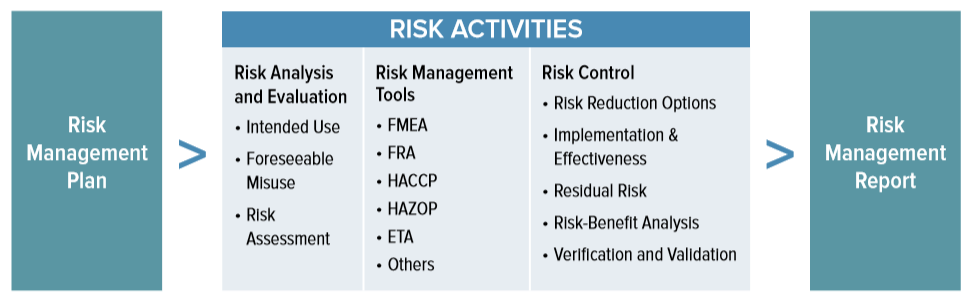 www.orielstat.com
www.orielstat.com
risk matrix
(PDF) Medical Device Risk Management - IsO 14971 - DOKUMEN.TIPS
 dokumen.tips
dokumen.tips
(PDF) RISK MANAGEMENT FOR MEDICAL DEVICES (EN ISO14971:2019) … · 2021
 dokumen.tips
dokumen.tips
The Big Picture For Medical Device Risk Management - Sunstone Pilot, Inc.
 sunstonepilot.com
sunstonepilot.com
risk evaluated testing pilot
Training Services For The Medical Device Industry - Risk Management
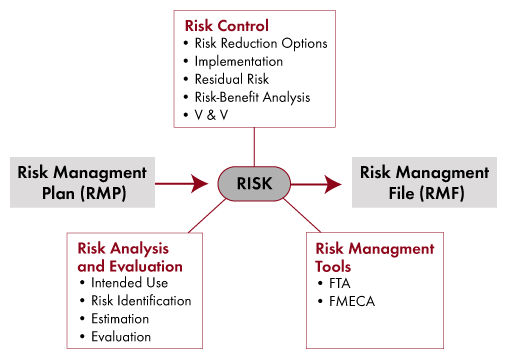 www.swqual.com
www.swqual.com
risk management medical device industry overview topics include covered requirements
Medical Device Risk Management, Assessment And Analysis
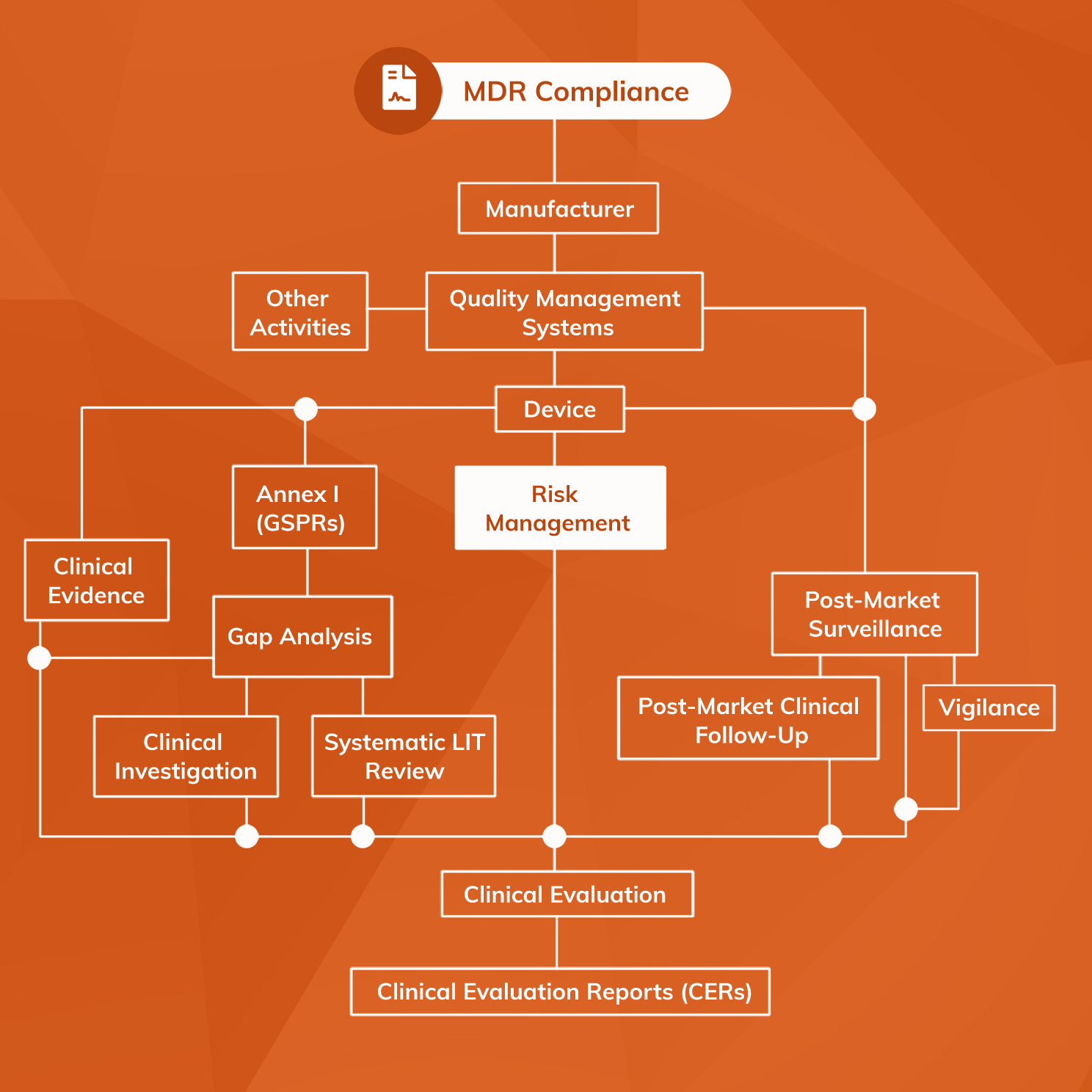 www.mantrasystems.co.uk
www.mantrasystems.co.uk
Creating A Medical Device Risk Management Plan And Doing Analysis
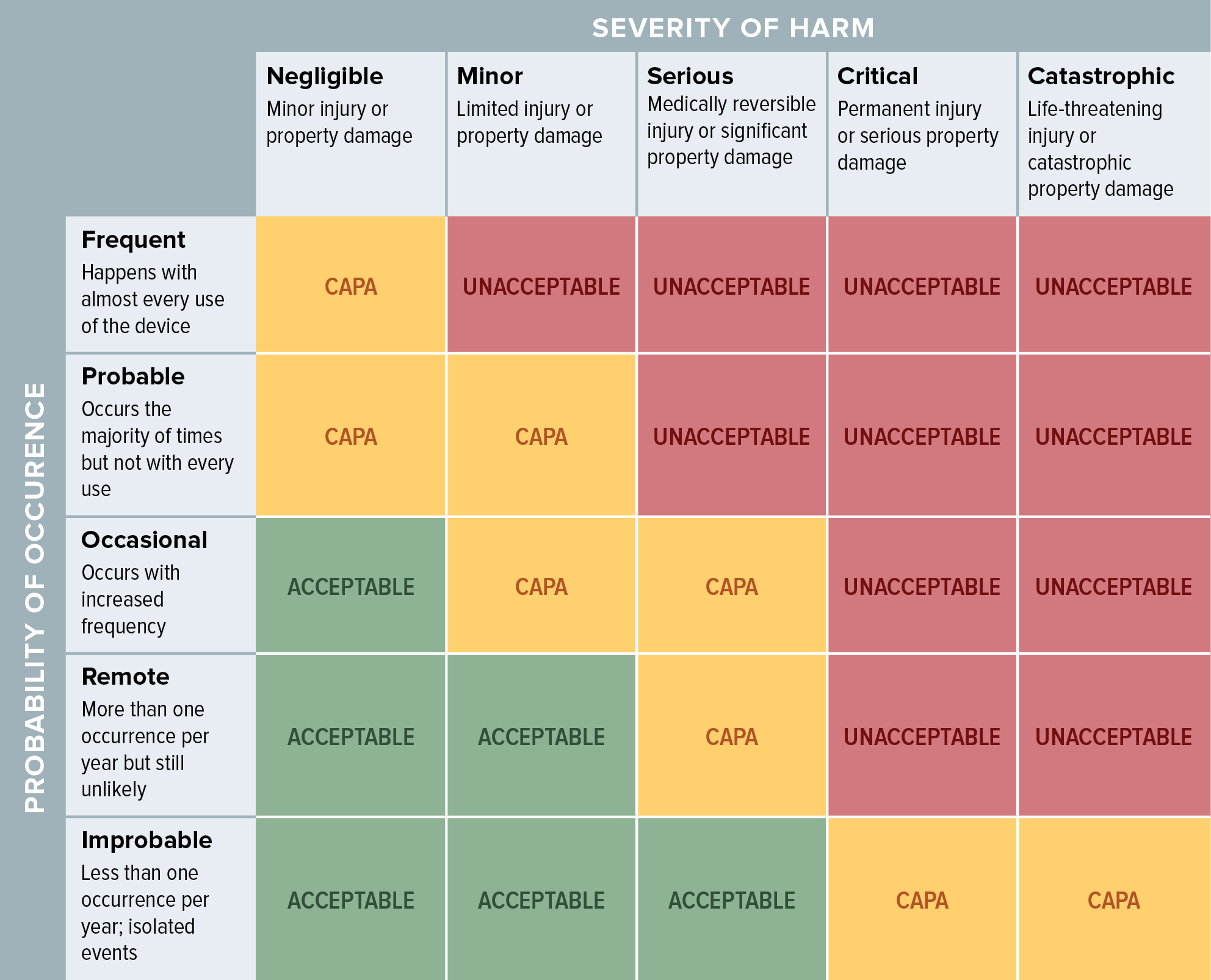 www.orielstat.com
www.orielstat.com
severity probability estimation driven
Risk medical device management report example template google twitter share examples. The big picture for medical device risk management. Medical device risk management, assessment and analysis